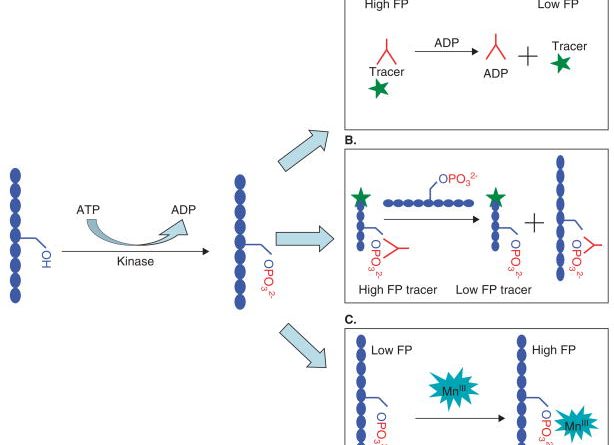
The challenge of selecting protein kinase assays for lead discovery optimization.
BACKGROUND: Protein kinases represent one of the most promising groups of drug targets owing to their involvement in such pathological conditions as cancer, inflammatory diseases, neural disorders, and metabolism problems.
In the last few years, numerous pharmaceutical and biotech companies have established kinase high-throughput screening (HTS) programs, and the reagent and service industries for kinase assay platforms, kits, and profiling services have begun to thrive.
OBJECTIVE: The plethora of different assay formats available today poses a great challenge to scientists who want to select a technology that will be cost efficient, convenient to use, and have low false positive and false negative rates.
METHODS: In the current review, we summarize the most commonly used kinase assay methods in the drug discovery process, present the advantages and disadvantages of each of these methods, and discuss the challenges of discovering kinase inhibitors by using these technologies.
CONCLUSIONS: The decision of selecting the assay formats for HTS or service platform for profiling should take into account not only the final goals of the screens but also the limitation of resources.
Innovative farmers and regulatory gatekeepers: Genetically modified crops regulation and adoption in developing countries.
The regulation of genetically modified (GM) crops is a topical issue in agriculture and environment over the past 2 decades. The objective of this paper is to recount regulatory and adoption practices in some developing countries that have successfully adopted GM crops so that aspiring countries may draw useful lessons and best practices for their biosafatey regulatory regimes.
The first 11 mega-GM crops growing countries each with an area of more than one million hectares in 2014 were examined. Only five out of the 11 countries had smooth and orderly adoption of these crops as per the regulatory requirement of each country. In the remaining 6 countries (all developing countries), GM crops were either introduced across borders without official authorization, released prior to regulatory approval or unapproved seeds were sold along with the approved ones in violation to the existing regulations. Rapid expansion of transgenic crops over the past 2 decades in the developing world was a result of an intense desire by farmers to adopt these crops irrespective of regulatory roadblocks.
Lack of workable biosafety regulatory system and political will to support GM crops encouraged unauthorized access to GM crop varieties. In certain cases, unregulated access in turn appeared to result in the adoption of substandard or spurious technology which undermined performance and productivity. An optimal interaction among the national agricultural innovation systems, biosafety regulatory bodies, biotech companies and high level policy makers is vital in making a workable regulated progress in the adoption of GM crops.
Factoring forgone opportunities to farmers to benefit from GM crops arising from overregulation into biosafety risk analysis and decision making is suggested. Building functional biosafety regulatory systems that balances the needs of farmers to access and utilize the GM technology with the regulatory imperatives to ensure adequate safety to the environment and human health is recommended.